Preservative Challenge Testing and USP 51
What does USP 51 cover?
USP 51 covers the effectiveness of antimicrobial preservatives in preventing microbial growth. Aqueous-based medical products contain antimicrobial preservatives. USP 51 defines aqueous as a formulation with a water activity of more than 0.6. Preservatives protect from microbial growth during the manufacturing process and the withdrawal of multiple doses from a vial, tube, or another product container. Indeed, it is expected that all multi-dose medical products requiring sterilization contain at least one preservative to combat microbial growth. All antimicrobial agents are toxic at high enough levels. Thus, it is essential to verify the minimum effective dose of preservative for a medical product. Five common microorganisms are described in USP 51 for preservative challenge testing. These five standard microorganisms are the minimum requirements for organism type and strain for preservative challenge testing. However, it is encouraged to test for any other supplemental species of microorganisms helpful in measuring the biological activity of a product’s preservative system.
How are USP 51 tests performed?
First, cultures of Candida albicans (ATCC No. 10231), Aspergillus brasiliensis (ATCC No. 16404),
Escherichia coli (ATCC No. 8739), Pseudomonas aeruginosa (ATCC No. 9027), and Staphylococcus aureus (ATCC No. 6538) are prepared. Stock cultures of these organisms are often created by centrifugation of an ATCC culture to collect all of the cells in one place, washing the previous culture to remove residual media of the prior culture, and resuspending the microorganisms in a sterile suspension fluid. The suspension fluids for each microorganism referenced above are prepared at a microbial count of about 1 × 108 colony-forming units per milliliter (CFU/mL).
Preservative efficacy testing is performed in five sterile, capped containers. Suppose the original container of a product under assessment is sterile, can be entered aseptically, and holds an appropriate volume of the product being tested. In that case, preservative challenge testing may use the original containers with the filled product. Each of the five product samples is injected with a test suspension of either Candida albicans, Aspergillus brasiliensis, Escherichia coli, Pseudomonas aeruginosa, or Staphylococcus aureus. Note that each product sample is exposed to only a single microbe of the five listed, and all microbes are exposed to the product during testing. The microbial test suspension injected is between 0.5%- 1% of the volume of the product under assessment and is at a concentration of 1 × 105 and 1 × 106 CFU/ml of the product for injectable, topical, and oral products. The final concentration per ml of product for antacid-like products is between 1 × 103 and 1 × 104 CFU/mL.
Each of the five product samples with their respective microbial injections is incubated at a simulated room temperature of 22.5 ± 2.5°C or 32.5 ± 2.5°C depending upon the microbe the product sample is exposed. Culturing conditions for the microbial preparations are detailed in Table 2 of USP 51, reproduced as Table 1 below.
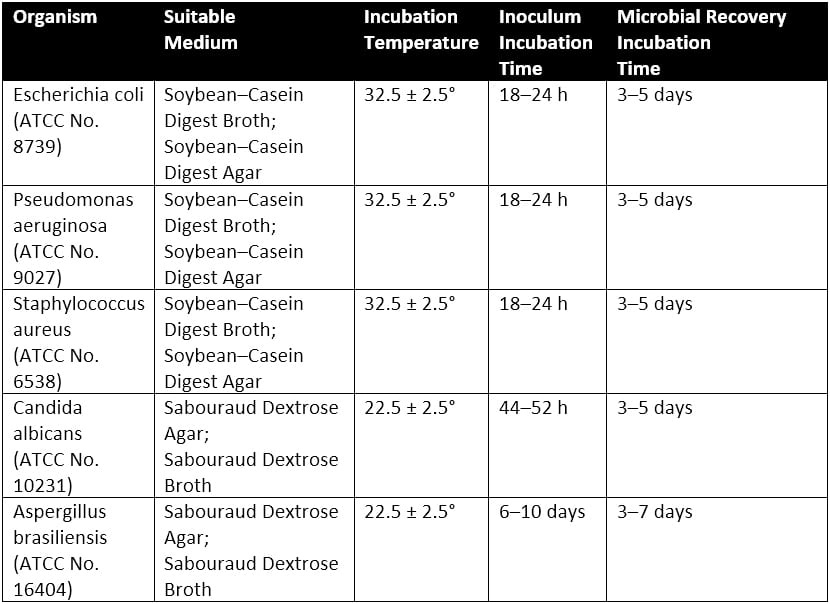
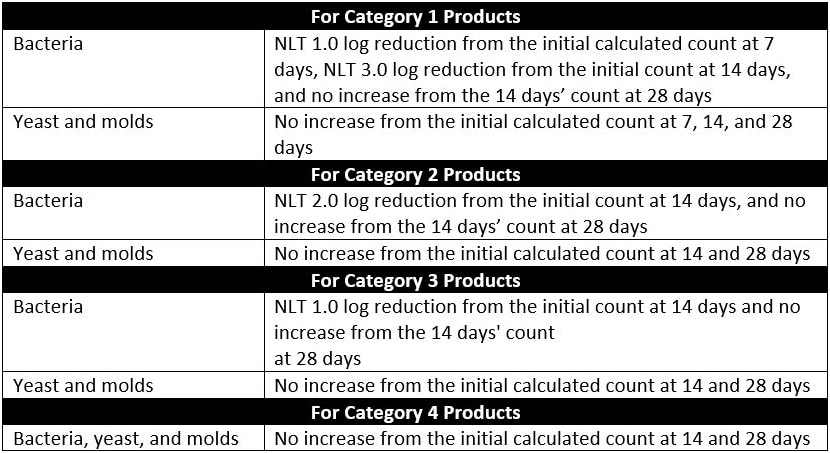
Microbial counts are taken at 7, 14, and 28 days for most microorganisms tested. The plate-count method is used to determine the number of CFU present in each of the inoculated product samples. This plate-count method is completed in duplicate. Once all counts are taken, the change in log10 of the concentration of CFU/ml is calculated for each microorganism. The requirements for antimicrobial effectiveness are met if no increase in microbial growth for the various organisms tested occurs within the timeframes specific to each organism are met. Table 3 of USP 51, reproduced as Table 2 below, details the criteria for tested microorganisms. Note that no increase in microbial growth is defined as not more than 0.5 log10 units more than the previous microbial growth value.
How does USP 51 relate to preservative challenge testing?
Preservatives are substances mixed into creams, gels, and other liquids. Preservatives are used to prevent the growth of bacteria, fungi, and other microorganisms within a medical, cosmetic, household, or food product. Preservatives also help keep the freshness of the appearance of a product and keep its consistency intact over time.
The USP 51 pharmacopeia methods outline the specifics of how the preservative challenge testing (also known as preservative efficacy testing) should be carried out. Specifically, USP 51 describes the preparation of test stains for challenge microorganisms, product sample preparation, and testing methodologies for preservative challenge testing. When testing in-house or outsourcing your testing to a contract testing facility, it is crucial to ensure the USP 51 pharmacopeia standards and methods are used for all preservative challenge testing.
MycoScience is a contract manufacturing organization specializing in sterile syringe and vial filling. MycoScience also offers Preservative Efficacy Testing, Sterilization Validations, Bioburden Testing, Cleaning Validations, Microbial Aerosol Challenge Testing, Accelerated Aging, Microbiology Testing, Cytotoxicity Testing, Bacterial Endotoxin Testing, EO Residual Testing, Package Integrity Testing & Environmental Monitoring services medical devices and allied industries. MycoScience is an ISO 13485 certified facility.
References
United States Pharmacopeial Convention. <51> Antimicrobial Effectiveness Testing. Rockville, MD, USA. 2021. (USPC <51>).
Sharing this in your social netwroks

