What Is Package Integrity Testing For Sterile Products
What is package integrity testing?
Suppose we break down the term package integrity. The definition of a package is a wrapped object or groups of objects. The state of integrity is a state of being whole and undivided. Thus, package integrity testing has to do with testing how well a package stays complete and undivided to keep wrapped objects protected and sterile. In the context of package integrity and package integrity testing, a product package is considered both the product packaging itself and the contents of the product.
What is a container-closure system?
A container–closure system includes primary packaging (packaging in direct contact with the product) and secondary packaging (packaging that supports package assembly such as an aluminum cap as a secondary seal for a stoppered vial). Container-closure systems for sterile product-package integrity must keep product contents within and environmental contaminants out. Types of packaging leaks of concern for sterile product-package systems are detailed in Table 1 of USP 1207, reproduced below.
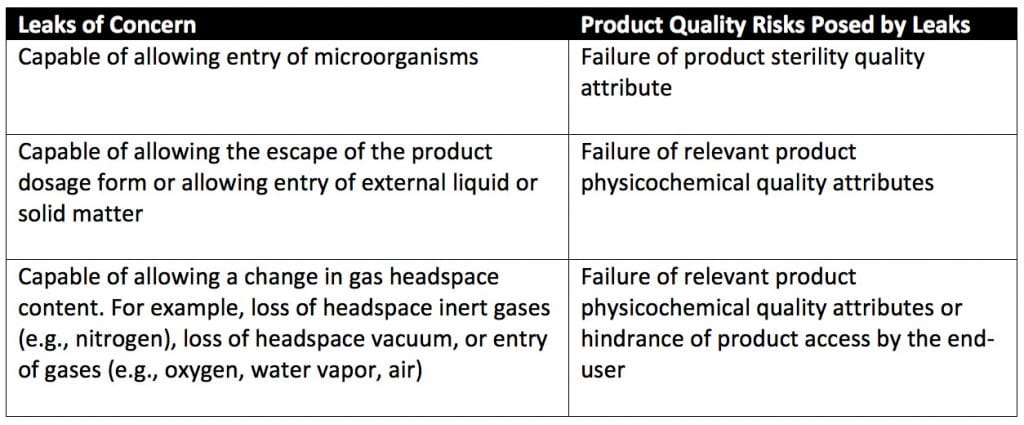
What are the differences between package integrity and container-closure integrity?
Package integrity and container-closure integrity are terms that are used interchangeably. These terms are the same, depending upon the definition of container-closure integrity. In the past, container–closure integrity referred to a package that had passed or was capable of passing a microbiological challenge test. With the USP 1207 definition of container-closure integrity, package integrity and container-closure integrity are synonymous. The USP 1207 guidance defines container–closure integrity as the absence of all package leaks that risk product quality. In other words, the package is intact such that any small leaks of concern are absent.
What are the differences between leakage and permeation for package integrity testing?
Leakage is a breach in packaging components that allows the accidental entry or escape of solids, fluids, or air. The presence, magnitude, and location of leaks within a packaging system are evaluated using various package integrity tests. Permeation is the passage of fluid (such as gas) in and out of a package’s wall. Permeation occurs due to material properties of the packaging material chosen and not from a hole or a breach in the packaging system. When it comes to non-porous packaging, non-porous packaging allows permeation but does not enable volumetric airflow. Package integrity tests are designed to evaluate packaging leaks, not packaging permeability.

What is package seal quality testing?
Package seal quality tests monitor the quality and consistency of a package’s seal and any factors that could influence a package’s ability to maintain its integrity. Seal strength testing is a standard packaging seal quality test and quality metric. While seal quality tests are not leak tests, they play a valuable role in monitoring the seal itself. Seal quality tests also characterize and monitor the package materials, the package components, and the processes required to create the packaging seal or closure mechanism. Seal quality testing is essential for package integrity quality validation throughout the product life cycle and should be performed regularly and if any alterations to the original packaging system occur.
What are the differences between package integrity and container-closure integrity?
MycoScience is a contract manufacturing organization specializing in sterile syringe and vial filling. MycoScience also offers Preservative Efficacy Testing, Sterilization Validations, Bioburden Testing, Cleaning Validations, Microbial Aerosol Challenge Testing, Accelerated Aging, Microbiology Testing, Cytotoxicity Testing, Bacterial Endotoxin Testing, EO Residual Testing, Package Integrity Testing & Environmental Monitoring services medical devices and allied industries. MycoScience is an ISO 13485 certified facility.
References
United States Pharmacopeial Convention. <1207> Package Integrity Evaluation- Sterile Products. Rockville, MD, USA. 2021. (USPC <1207>).
Sharing this in your social netwroks

